- Mefloquine
-
Mefloquine 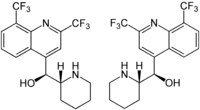
Systematic (IUPAC) name (R*,S*)-2,8-bis(trifluoromethyl)quinolin-4-yl]-(2-piperidyl)methanol Clinical data Pregnancy cat. C (U.S.) Legal status ? Routes oral Pharmacokinetic data Metabolism Extensively hepatic; main metabolite is inactive Half-life 2 to 4 weeks Excretion Primarily bile and feces; urine (9% as unchanged drug, 4% as primary metabolite Identifiers CAS number 53230-10-7 
ATC code P01BC02 PubChem CID 40692 DrugBank DB00358 ChemSpider 37171 
UNII TML814419R 
KEGG D04895 
ChEMBL CHEMBL416956 
Chemical data Formula C17H16F6N2O Mol. mass 378.312 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Mefloquine hydrochloride (also known as Lariam or Mefaquin) is an orally administered medication used in the prevention and treatment of malaria. Mefloquine was developed in the 1970s at the United States Department of Defense's Walter Reed Army Institute of Research as a synthetic analogue of quinine. The brand name drug, Lariam, is manufactured by the Swiss company Hoffmann–La Roche. In August 2009, Roche stopped marketing Lariam in the United States. Generic mefloquine from other manufacturers, is still widely available. Rare but serious neuropsychiatric problems have been associated with its use.
Contents
Medical uses
Mefloquine is used to both prevent and treat certain forms of malaria.[1]
Malaria prevention
Mefloquine is useful for the prevention of malaria in all areas except for those where parasites may have resistance to multiple drugs.[2] It is typically taken for one to two weeks before entering an area with malaria.[1] Doxycycline and atovaquone/proguanil provide protection within one to two days and may be better tolerated.[3][4] If a person becomes ill with malaria despite prophylaxis with mefloquine the use of halofantrine and quinine for treatment may be ineffective.[5]
Malaria treatment
Once a person has contracted malaria, mefloquine is recommended as a second line treatment for chloroquine sensitive or resistant falciparum malaria and is deemed a reasonable alternative for uncomplicated chloroquine resistant vivax malaria.[1][5]
It is not recommended for severe malaria infections, particularly infections from P.falciparum which should be treated with intravenous antimalarials.[1][5] Mefloquine does not eliminate parasites in the liver phase of the disease, and people with P. vivax malaria should be treated with a second drug that is effective for the liver phase, such as primaquine.[6]
In pregancy and breastfeeding
The World Health Organization gives approval for the use of mefloquine in the 2nd and 3rd trimester of pregnancy and use in the 1st trimester does not mandate termination of pregnancy.[2] Women should however not become pregnant and should use effective birth control while taking mefloquine.[7] It may be used during breastfeeding, though the drug appears in breast milk in low concentrations.[2][8]
Adverse effects
Mefloquine is contraindicated in those with a previous history of seizures or a recent history of psychiatric disorders.[1] Severe side effects requiring hospitalization are rare.[2] Rates of side effects appear similar to other medications used for malaria prevention.[3]
Neuropsychiatric
Neuropsychiatric effects are reported with mefloquine use.[1] It is not known if this is a causal relationship.[1][2] The FDA product guide states it can cause mental health problems including: anxiety, hallucinations, depression, unusual behavior, and suicidal ideations among others.[7] Some have reported severe central nervous system events requiring hospitalization in about 1:10,000 people taking mefloquine for malaria prevention with milder events (e.g., dizziness, headache, insomnia, and vivid dreams) in up to 25%.[9] When some measure of subjective severity is applied to the rating of adverse events, about 11-17% of travelers are incapacitated to some degree.[3]
Pneumonitis
The FDA has reported an association with pneumonitis and eosinophilic pneumonia[10]
Cardiac
Mefloquine may cause abnormalities with heart rhythms that are visible on electrocardiogram. Combining mefloquine with other drugs that cause similar effects, such as quinine or quinidine, can increase these effects. Combining mefloquine with halofantrine can cause significant increases in QTc interval.[11]
Mechanism of action
Mefloquine blocks the action of the chemical that the parasites produce to protect themselves once inside the red blood cells. There is increasing resistance to this drug however, Is costly to produce and also has serious side effects.
Elimination
Mefloquine is metabolized primarily through the liver. Elimination of mefloquine in anyone with impaired liver function may be prolonged, resulting in higher plasma levels and an increased risk of adverse reactions. The mean elimination plasma half-life of mefloquine is between 2 and 4 weeks. Total clearance is through the liver and the primary means of excretion is through the bile and feces as opposed to only 4% to 9% excreted through the urine. During long-term use, the plasma half-life remains unchanged.[12][13]
Liver function tests should be performed during long-term administration of mefloquine.[14] Alcohol use should be avoided during treatment with mefloquine.[15]
Chirality
Mefloquine is a chiral molecule with two asymmetric carbon centres, which means it has four different stereoisomers. The drug is currently manufactured and sold as a racemate of the (R,S)- and (S,R)-enantiomers by Hoffman-LaRoche, a Swiss pharmaceutical company. Essentially it is two drugs in one. Plasma concentrations of the (-)-enantiomer are significantly higher than those for the (+)-enantiomer and the pharmokinetics between the two enantiomers are significanly different. The (+)-enantiomer has a shorter half-life than the (–)-enantiomer.[3]
According to some research,[16] the (+)-enantiomer is more effective in treating malaria, and the (–)-enantiomer specifically binds to adenosine receptors in the central nervous system, which may explain some of its psychotropic effects. It is not known whether mefloquine goes through stereoisomeric switching in vivo.
History
Mefloquine was invented at Walter Reed Army Institute of Research (WRAIR) in the 1970s shortly after the end of the Vietnam war where 1% of US combat troops contracted malaria every day. Mefloquine was number 142,490 out of a total of 250,000 antimalarial compounds screened during the study.[17]
Mefloquine was the first Public-Private Venture (PPV) between the US Department of Defense and a pharmaceutical company. WRAIR transferred all its phase I and phase II clinical trial data to Hoffman LaRouche and Smith Kline. FDA approval as a treatment for malaria was swift. Most notably, phase III safety and tolerability trials were skipped.[17]
However, mefloquine was not approved by the FDA for prophylactic use until 1989. This approval was based primarily on compliance while safety and tolerability were overlooked.[17] Because of the drug's very long half-life, the Centers for Disease Control originally recommended a mefloquine dosage of 250 mg every two weeks; however, this caused an unacceptably high malaria rate in the Peace Corps volunteers that participated in the approval study, so the drug regimen was switched to once a week.[3]
The first randomized controlled trial on a mixed population was first performed in 2001. Roughly 67% reported greater than or equal to 1 adverse event with 6% of the users reporting severe events requiring medical attention.[17]
With this kind of data, it is certain the FDA and other international licensing authorities would not have approved mefloquine for prophylactic use.[17]
There have also been no studies on the effects of co-administration of mefloquine and other drugs. The fatal drug reactions that ensued might have been a result of this lack of knowledge of possible contraindications. Trials in the 1990s and early 2000s verified mefloquine's neurotoxicity and significant potential for neuropsychiatric side effects.[17]
Post marketing data was easily discounted as anecdotal and "media hype" by the US Army researchers and travel medicine practitioners.[17] Since the side effects that mefloquine can cause have not been fully defined, and there appears to be no incentive for the current manufacturers to further investigate mefloquine, the drug may be discarded.[17] As evidence, the US military dropped mefloquine as its primary antimalarial in 2009.[18][19]
Society and culture
United States military
On 2 February 2009, Lieutenant General Eric Schoomaker, Army Surgeon General, issued the following directive:
"In areas where doxycycline and mefloquine are equally efficacious in preventing malaria, doxycycline is the drug of choice. Mefloquine should only be used for personnel with contraindications to doxycycline and who do not have any contraindications to the use of mefloquine . . . . Mefloquine should not be given to soldiers with recent history of Traumatic Brain Injury (TBI) or who have symptoms from a previous TBI. Malarone would be the treatment of choice for these soldiers who cannot take doxycycline or mefloquine."[19]
The following September, Hon. Ellen Embry, then Acting Assistant Secretary of Defense for Health, issued the same policy making doxycycline the antimalarial of choice across all the US armed services.[18]
The 2012 CDC travel yellow book was recently amended to include a section "special considerations for US military deployments". It makes doxycycline the primary anti-malarial prophylactic to be used for all military deployments. As a note of historical significance, Col Alan Magill and Col Robert Defriates co-authored to this section.[20] Both were the primary promoters for the continued use of mefloquine by the US military in the late 1990s and early 2000s. Magill is the former commanding officer of the drug research unit at WRAIR that performed the studies to find a safer version of mefloquine. Defraites chaired the investigation into the 2002 murder/suicides at Ft. Bragg. There 4 soldiers were accused of murdering their wives; two of these soldiers committed suicide. Their actions as well as other military suicides have been linked to Lariam use[21][22].
Research
In June 2010, the first case report appeared of a PML being successfully treated with mefloquine. Mefloquine is an antimalarial drug that can also act against the JC virus. Administration of mefloquine seemed to eliminate the virus from the patient's body and prevented further neurological deterioration.[23]
WRAIR has published several papers outlining ongoing efforts at that institution to make mefloquine safer by producing a drug composed of only the (+)-enantiomer.
References
- ^ a b c d e f g "Lariam". The American Society of Health-System Pharmacists. http://www.drugs.com/monograph/lariam.html. Retrieved 3 April 2011.
- ^ a b c d e Schlagenhauf, P; Adamcova, M, Regep, L, Schaerer, MT, Rhein, HG (2010-12-09). "The position of mefloquine as a 21st century malaria chemoprophylaxis.". Malaria journal 9: 357. doi:10.1186/1475-2875-9-357. PMID 21143906.
- ^ a b c d e Schlagenhauf, P. (1999). "Mefloquine for malaria chemoprophylaxis 1992-1998". Travel Med 6 (2): 122–123. PMID 10381965.
- ^ Jacquerioz, FA; Croft, AM (2009-10-07). "Drugs for preventing malaria in travellers.". Cochrane database of systematic reviews (Online) (4): CD006491. doi:10.1002/14651858.CD006491.pub2. PMID 19821371.
- ^ a b c "Lariam medication guide". Hoffman La Roche. p. 4. http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/019591s023lbl.pdf. Retrieved 14 April 2011.
- ^ "Lariam medication guide". p. 4. http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/019591s023lbl.pdf.
- ^ a b http://www.fda.gov/downloads/Drugs/DrugSafety/ucm088616.pdf
- ^ "Lariam medication guide". Hoffman La Roche. p. 9. http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/019591s023lbl.pdf. Retrieved 14 April 2011.
- ^ AlKadi, HO (2007). "Antimalarial drug toxicity: a review". Chemotherapy 53 (6): 385–91. doi:10.1159/000109767. PMID 17934257.
- ^ "Postmarketing Reviews - Volume 1, Number 4, Summer 2008". U.S. Food and Drug Administration. 2008. http://www.fda.gov/Drugs/DrugSafety/DrugSafetyNewsletter/ucm120613.htm.
- ^ "Lariam medication guide". p. 10. http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/019591s023lbl.pdf.
- ^ "Lariam product monogram". Hoffman La Roche Limited. p. 3. http://www.rochecanada.com/fmfiles/re7234008/Research/ClinicalTrialsForms/Products/ConsumerInformation/MonographsandPublicAdvisories/Lariam/lariamMar3PME.pdf. Retrieved 24 April 2011.
- ^ "Lariam product monogram". Hoffman La Roche Limited. p. 4. http://www.rochecanada.com/fmfiles/re7234008/Research/ClinicalTrialsForms/Products/ConsumerInformation/MonographsandPublicAdvisories/Lariam/lariamMar3PME.pdf. Retrieved 24 April 2011.
- ^ "Lariam product monogram". Hoffman La Roche Limited. p. 6. http://www.rochecanada.com/fmfiles/re7234008/Research/ClinicalTrialsForms/Products/ConsumerInformation/MonographsandPublicAdvisories/Lariam/lariamMar3PME.pdf. Retrieved 24 April 2011.
- ^ "Lariam product monogram". Hoffman La Roche Limited. p. 18. http://www.rochecanada.com/fmfiles/re7234008/Research/ClinicalTrialsForms/Products/ConsumerInformation/MonographsandPublicAdvisories/Lariam/lariamMar3PME.pdf. Retrieved 24 April 2011.
- ^ Fletcher, A., and Shepherd, R. Use of (+)-mefloquine for the treatment of malaria. US 6664397.
- ^ a b c d e f g h Croft, AM (2007). "A lesson learnt: the rise and fall of Lariam and Halfan". J R Soc Med. 4 (4): 170–4. doi:10.1258/jrsm.100.4.170. PMC 1847738. PMID 17404338. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1847738.
- ^ a b http://www.lariaminfo.org/pdfs/policy-memo-secy-defense%20malaria-prophylaxis.pdf
- ^ a b http://www.pdhealth.mil/downloads/DASG_Memorandum.pdf
- ^ http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-8-advising-travelers-with-specific-needs/special-considerations-for-us-military-deployments.htm
- ^ [1]
- ^ http://www.military.com/NewsContent/0,13319,FL_lariam_022604,00.html
- ^ Gofton TE, Al-Khotani1 A, O'Farrell B, Ang LC, McLachlan RS (2010). "Mefloquine in the treatment of progressive multifocal leukoencephalopathy". J Neurol Neurosurg Psychiatry 82 (4): 452–455. doi:10.1136/jnnp.2009.190652. PMID 20562463. http://jnnp.bmj.com/content/early/2010/06/19/jnnp.2009.190652.full.
External links
- Manufacturer's information
- Mefloquine (Lariam) Action, Clearinghouse for information on mefloquine news, research, toxicity
- Controversies and Misconceptions in Malaria Chemoprophylaxis for Travelers
- The position of mefloquine as a 21st century malaria chemoprophylaxis
Antiparasitics – antiprotozoal agents – Chromalveolate antiparasitics (P01) Alveo-
lateIndividual
agentsOtherDHFR inhibitors
(antifols)Sulfadoxine • sulfamethoxypyrazineCoformulationFansidar# (sulfadoxine/pyrimethamine)OtherCombi-
nationsFixed-dose (coformulated) ACTsartemether-lumefantrine#
artesunate-amodiaquine (ASAQ)
artesunate-mefloquine (ASMQ)
dihydroartemisinin-piperaquine
artesunate-pyronaridineOther combinations
(not co-formulated)artesunate/SP • artesunate/mefloquine •
quinine/tetracycline • quinine/doxycycline • quinine/clindamycinHetero-
kontCategories:- Antimalarial agents
- Quinolines
- Piperidines
- Organofluorides
- World Health Organization essential medicines
Wikimedia Foundation. 2010.